NO
YES
YES
YES
Outpatient
treatment
options not
authorized or
recommended.
Supportive care
only.
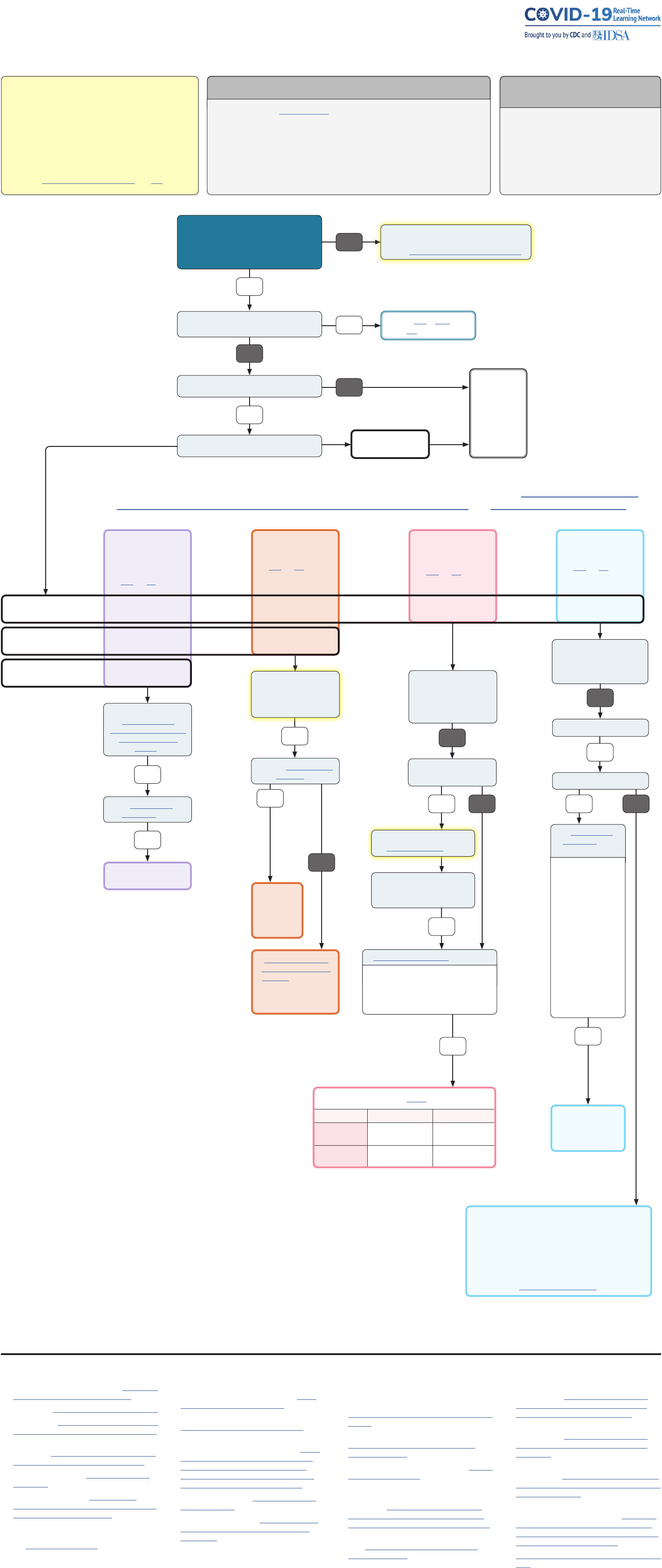
COVID-19 OUTPATIENT TREATMENT
GUIDELINES ROADMAP
Last Updated: February 2, 2023
This resource is intended to serve as a guide on available outpatient
COVID-19 treatment options, with links to FDA Emergency Use
Authorization information and guideline recommendations from
national guideline-developing organizations, where available. It is
not intended to endorse or otherwise promote a specific clinical
recommendation or course of action. Additionally, it does not
include other forms of guidance that may be available for specific
subsets of populations. Finally, the guidelines referenced here may
not consider local allocation and availability of scarce resources.
Additional information on where to access these therapeutics can be
found at the National Infusion Center Association
15
and HHS.
11
How many days since symptom onset?
Does your patient have any symptoms?
Does your patient
have COVID?
Positive results of direct SARS-CoV-2 testing
YES
> 8 DAYS
See CDC
1
/ IDSA
3
/
NIH
2
guidance.
NO
Is your patient hospitalized for COVID-19 or
requiring increased O
2
for COVID-19?
NO
Risk factors for severe COVID-19
10
Included here are some medical conditions that may place patients at a higher risk for progression
to severe COVID-19:
• Age 65 years and older
• BMI of more than 25 kg/m
2
• Pregnancy
• Chronic kidney disease
• Diabetes mellitus
• Immunosuppressing
medications
• Cardiovascular disease or
hypertension
• Chronic lung disease
• Sickle cell disease
• Neurodevelopmental
disorders or conditions that
confer medical complexity
• Medical technological
dependence, e.g.,
tracheostomy
When giving products under Emergency
Use Authorization, providers must:
1. Give patient fact sheet for patients.
2. Inform patient of alternatives to treatment.
3. Inform patient that this is an unapproved drug.
References
1. CDC guidelines for clinical management: Management
of Patients with Confirmed 2019-nCoV | CDC
2. NIH guidelines: COVID-19 Treatment Guidelines (nih.gov)
3. IDSA guidelines: IDSA Guidelines on the Treatment and
Management of Patients with COVID-19 (idsociety.org)
4. NIH Guidelines Panel’s Statement on Drug Interactions
with Paxlovid: Statement on Paxlovid Drug-Drug Inter-
actions | COVID-19 Treatment Guidelines (nih.gov)
5. Sotrovimab EUA fact sheet: SOTROVIMAB-EUA.PDF
(gskpro.com)
6. Molnupiravir EUA fact sheet: FACT SHEET FOR
HEALTHCARE PROVIDERS: EMERGENCY USE AUTHORI-
ZATION FOR MOLNUPIRAVIR (fda.gov)
7. Remdesivir (Veklury
TM
) Package Insert (Prescribing
Information) with the extended approval for outpatient
use: veklury_pi.pdf (gilead.com)
8. Convalescent Plasma EUA fact sheet and List of Tests
and Cutoffs of High Titers (table on page 9): https://
www.fda.gov/media/141477/download
9. Paxlovid EUA Fact Sheet for Healthcare Providers:
https://www.fda.gov/media/155050/download
10. CDC List of Medical Conditions with risk of progression
to severe COVID-19: Patient-directed format: People
with Certain Medical Conditions | CDC Healthcare
provider format: Underlying Medical Conditions
Associated with Higher Risk for Severe COVID-19:
Information for Healthcare Providers (cdc.gov)
11. HHS Therapeutics Locator: COVID-19 Therapeutics
Locator (arcgis.com)
12. Bebtelovimab EUA fact sheet: Fact Sheet for Health-
care Providers: Emergency Use Authorization for
Bebtelovimab
13. Remdesivir EUA fact sheet for use in CHILDREN weigh-
ing 3.5 kg to <40 kg OR those less than 12 years old
weighing at least 3.5 kg, who are NOT HOSPITALIZED:
EUA 046 Veklury (remdesivir) FS for HCPs (01212022)
(fda.gov)
14. Combat COVID Website on Treatment Options:
COVID-19 Resources For Healthcare Providers |
combatCOVID.hhs.gov
15. National Infusion Center Association Website: National
Infusion Center Association. Relates to the Access and
Availability link.
16. FDA CDER Scientific Review Documents for COVID-19
Related EUAs: CDER Scientific Review Documents
Supporting Emergency Use Authorizations for Drug
and Biological Therapeutic Products | COVID-19 | FDA
17. University of Liverpool COVID-19 Drug Interactions
Page: https://www.covid19-druginteractions.org/
prescribing_resources
18. NIH guidance on therapies for high-risk non-hos-
pitalized patients: https://www.covid19treatment-
guidelines.nih.gov/therapies/statement-on-thera-
pies-for-high-risk-nonhospitalized-patients/
19. IDSA guidance on nirmatrelvir/ritonavir (Paxlovid)
and molnupiravir: https://www.idsociety.org/prac-
tice-guideline/covid-19-guideline-treatment-and-
management
20. Early Use of High-titer Convalescent Plasma in
Outpatients Trial: Randomized Controlled Trial of Early
Outpatient COVID-19 Treatment with High-Titer Conva-
lescent Plasma (nih.gov)
21. IDSA guidance on outpatient use of convalescent
plasma in immunosuppressed patients: https://www.
idsociety.org/globalassets/idsa/practice-guidelines/
covid-19/treatment/idsa-covid-19-gl-tx-and-mgmt---
convalescent-plasma-2022-02-03.pdf
22. FDA updates Sotrovimab emergency use authorization
| FDA
References
20. IDSA guidance on nirmatrelvir/ritonavir (Paxlovid)
and molnupiravir: https://www.idsociety.org/prac-
tice-guideline/covid-19-guideline-treatment-and-
management
21. Early Use of High-titer Convalescent Plasma in
Outpatients Trial: Randomized Controlled Trial of Early
Outpatient COVID-19 Treatment with High-Titer Conva-
lescent Plasma (nih.gov)
22. IDSA guidance on outpatient use of convalescent
plasma in immunosuppressed patients: https://www.
idsociety.org/globalassets/idsa/practice-guidelines/
covid-19/treatment/idsa-covid-19-gl-tx-and-mgmt---
convalescent-plasma-2022-02-03.pdf
23. FDA updates Sotrovimab emergency use authorization
| FDA
Molnupiravir
17,18
(Lagevrio®)
See IDSA and NIH guidelines.
*Positive test not required;
only diagnosis of COVID-19
Due to embryofetal toxicity in animals, molnupiravir is not
recommended for use in pregnancy.
If the decision is made to use molnupiravir in pregnancy, the
prescriber must document that potential benefits and risks of
molnupiravir use in pregnancy from the EUA factsheet were
discussed with the patient, and the patient was made aware of
Merck’s pregnancy surveillance program at 1-877-888-4231 or
pregnancyreporting.msd.com.
NO
YES
YES NO
Is your patient age 18 or older?
Is pregnancy ruled out?
Is Emergency Use
Authorization
6
met?
Including:
• At high risk for
progression to severe
COVID-19, including
hospitalization or death
• People of childbearing
potential: Should use
reliable contraception
for the duration of
molnupiravir treatment
& for 4 days after it is
stopped
• Men: If sexually
active, should use a
reliable method of
contraception during
molnupiravir treatment
& for 3 months after
molnupiravir is stopped
Are alternative COVID-19
treatment options approved or
authorized by FDA accessible or
clinically appropriate?
•
YES
Dosing: 250 mL high-titer
convalescent plasma
8
Does patient take
immunosuppressive
medications or have evidence
of immunosuppressive
disease?
21
Is Emergency Use
Authorization met?
8
YES
High-titer
convalescent
plasma
8, 20
See IDSA and NIH guidelines.
•
•
•
YES
Dosing:
200 mg IV on
day 1,
100 mg IV on
days 2 & 3
YES
NO
Dosing for children less
than 12 years and <40kg
and ≥3 kg: 5 mg/kg IV on
day 1 followed by 2.5 mg/
kg IV once daily from day 2
to day 3
Is patient over 12 years OR
over 40 kg?
7, 13
Does patient have access
to outpatient infusion or
inpatient admission for
administration of remdesivir?
Remdesivir*
7, 13
(Veklury®)
See IDSA and NIH guidelines.
*Positive test not required;
only diagnosis of COVID-19
•
•
© 2023. Infectious Diseases Society of America. Reprinted with permission.
This resource was funded in part by a cooperative agreement with the Centers for Disease Control and Prevention (grant number NU50CK000574). The Centers for Disease Control and Prevention is an agency within the
Department of Health and Human Services (HHS). The contents of this resource do not necessarily represent the policy of CDC or HHS, and should not be considered an endorsement by the Federal Government.
YES
YES
NO
YES
Nirmatrelvir/
ritonavir
17,18
(Paxlovid™)
See IDSA and NIH guidelines.
*Positive test not required;
only diagnosis of COVID-19
Does patient have severe
renal impairment
(eGFR <30 mL/min) OR
severe hepatic impairment
(Child-Pugh class C)?
Check for drug interactions using
evidence-based tools
17, 18, 19
Is the patient taking any other
medications?
Can these medicines be held,
dose-adjusted, or substituted
while Paxlovid
™
course is given?*
Is Emergency Use Authorization
9
met? Including:
• Age ≥ 12 years
• Weight ≥ 40 kg
• At high risk for progression to severe
COVID-19, including hospitalization or death
NO
•
Paxlovid
™
Dosing:
9
GFR Nirmatrelvir Ritonavir
≥60 mL/min 300 mg 2x daily for
5 days
100 mg 2x daily for
5 days
30 to <60 mL/
min
150 mg 2x daily for
5 days
100 mg 2x daily for
5 days
*hold prohibited medicines starting 12 hours before Paxlovid
™
and reinitiate 3-5 days after final Paxlovid dose
Dosing: Molnupiravir
800 mg (four 200 mg
capsules) orally twice
daily for 5 days
≤ 8 DAYS
≤ 5 DAYS
≤ 7 DAYS
This roadmap is not intended to represent a prioritization scheme for therapeutic choices.
For information on prioritization of one outpatient treatment over another, see NIH’s guidelines on the
therapeutic management of non-hospitalized adults with COVID-19 and ASPR Clinical Decision Aid.
There are no currently approved monoclonal Abs
available in the U.S. for pre-exposure prophylaxis for
COVID; vaccination and boosting is recommended.
